$BNTX | BioNTech: mRNA therapies beyond the COVID-19 vaccine
An exciting approach to auto-immune conditions published in Science shows the broad potential of future mRNA therapies
In January of 2021, BioNTech released a press release highlighting work that its research teams and collaborators at the University of Gutenberg published in Science1 showing the potential for an mRNA vaccine to eliminate auto-immune disease related to multiple sclerosis (MS). This news came at a time before emergency authorization or approval of its COVID-19 vaccine, which we now know works very well for inducing production of SARS-COV-2 spike protein, training the body to recognize and eliminate the pathogen. Similar approaches are now being explored in other immune applications by a myriad of firms to treat infectious disease and cancer. However, BioNTech’s published approach to auto-immune conditions showcases how mRNA therapeutic technology has potential beyond inducing antigenic immune response to foreign pathogens. BioNTech’s early success with their COVID-19 vaccine, sizeable revenues in 2021 and beyond, and deep partnerships with academia, Germany, and Pfizer may give them greater latitude to take risks in developing novel mRNA therapies beyond obvious indications, and we believe the Science paper also illustrates the firm’s scientific creativity and culture of rapid innovation.
BioNTech’s scientific teams have gained a significant amount of experience developing mRNA technology to fight COVID-19. They were originally developing mRNA vaccine technology for influenza virus before the pandemic. Their expertise with a highly temperamental technology represents an almost artistic mastery of the science of translation: they needed to develop an injectable messenger RNA formulation, complete with the optimal code and delivery vehicle, that tricks the body’s own protein manufacturing machinery to produce a desired protein instead of being altered or silenced by epigenetic regulatory processes. The history of the technology, its dysfunctional cast of characters, and its well-populated graveyard of commercial biotech ventures is covered well in this Nature news feature.
The takeaway message here is that BioNTech has overcome many of the challenges related to successful implementation of mRNA therapy and this most likely gives them a major advantage in expanding into other applications.
Over a year later, it is a perfect time to revisit the Science article in support of the argument that BioNTech is ahead in the race to apply mRNA technology to treat pesky autoimmune diseases, which account for 4 of the top 10 grossing drugs of all time. The research talent and creative approach exemplified in the academic paper along with the inherent advantages of mRNA therapy (e.g. dosage, tolerability, speed, and unit cost) could mean BioNTech may soon have the opportunity to steal the ball from established mAbs in auto-immune therapy, displacing cash cows like Humira or Enbrel while at the same time expanding into poorly treated diseases. Their approach supports the argument that they have the necessary mRNA expertise to tackle other diseases like cancer, and helps answer calls to use their vaccine war chest to acquire other firms as opposed to funding internal R&D. BioNTech makes a compelling case to stick to their core area of expertise (in addition to other robust programs in antibodies, small molecules, etc.). Our opinion is that the majority of a strategic moat in mRNA technology comes from the experience of scientific personnel and manufacturing processes.
To say this another way: there is a reason that BioNTech was first to a COVID-19 vaccine, and we expect this to become a pattern.
The published data on their auto-immune vaccine represents a potentially exciting breakthrough in the immunology field, utilizing an approach that, on the surface, looks very similar to mRNA vaccines against infectious disease, but upon closer examination of the cell immunology nuance, takes advantage of a slightly different MOA within the host’s immune system. Overall, the vision of the future that we see in BioNTech’s public work seems very promising.
BIONTECH’s Scientific Approach
The study2 begins by establishing a method for delivering an auto-antigen, which indicates a “self” protein that the body’s immune system erroneously recognizes as “not-self” in a way that prevents an inflammatory immune reaction. When these antigen proteins are successfully delivered to CD11+ lymphocytes in the absence of an inflammatory, cytokine-rich environment the immune system naturally expands T-regulatory cell populations specific to this endogenous protein, specifically lymphoid CD4+ antigen-presenting-cells (APCs), rather than activating toll-like-receptors (TLRs) that recruit CD11+ APCs and lymphocytes and trigger release of IFN-ɑ, which indicate to the immune system that a foreign invader is present and must be fought, including inducing antibody production. The population of these CD4+ T-regulatory cells increases and they migrate throughout tissue in the body where the auto-antigen is present. Once they are trained to recognize the auto-antigen, they act to suppress both adaptive and innate immune system cells that recognize the auto-antigen, preventing them from attacking the body’s healthy cells.
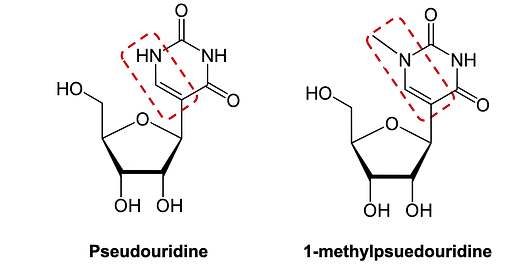
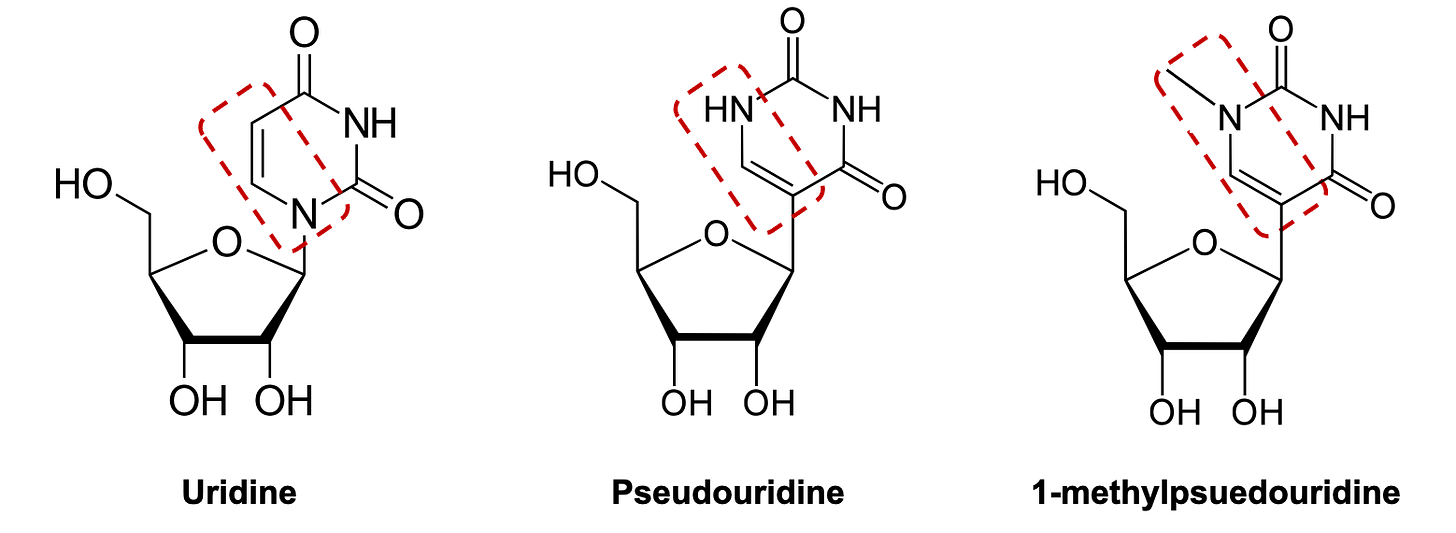
Why is the mRNA that they have designed capable of promoting T-reg production rather than yielding an inflammatory immune reaction typical for synthetic mRNA or the auto-antigen? The authors accomplish this using a method that was pioneered by Katalin Karikó and others at UPenn and was essential to the success of both the BioNTech and Moderna COVID-19 vaccines, inspired by the body’s own tRNA, replacing uridine (U)3 bases with a pseudouridine (ψ) base instead (see figure below). BioNTech (and Moderna’s) COVID-19 vaccines and approach to an auto-immune vaccine go a step further, using 1-methylpseudouridine (m1ψ) based on published data showing this additional base modification further reduces immunogenicity of synthetic mRNA via action on TLRs, increasing protein expression and minimizing inflammatory response. 4 5 6
The belief here is that the U bases serve a role in recognition of mRNAs by T-lymphocytes, the sentinels of the adaptive immune system that destroy foreign pathogens or molecules, often before they can produce protein (e.g. in the case of a viral infection). In the case of the COVID-19 vaccine, a mRNA that contains U rather than ψ or m1ψ would be rapidly degraded and therefore would never produce enough foreign protein to be learned by the adaptive immune system. The same translation mechanism for producing protein from mRNA holds in BioNTech’s auto-immune application, except unlike SARS-COV-2’s spike protein, the body has already developed adaptive immune response to the endogenous antigen. In the case of the auto-immune vaccine then, the goal is to induce production of protein that resembles the auto-antigen but avoids an inflammatory immune response, providing the necessary conditions for training the body’s regulatory immune cells to selectively deactivate that adaptive immune response when they encounter the auto-antigen. Exposure to the mRNA-derived, “de-clawed” antigen effectively “trains” the immune system to tolerate or “stand down” when it encounters the endogenous antigen otherwise responsible for development of an auto-immune disease.
Suppression of autoimmune response and expansion of T-regulatory cell populations validated in mouse disease models
The Authors show that when wild-type U-rich antigen mRNA is activated in the animals after vaccination (as the researchers would do if they were generating mice with a model of EAE, or MS-like, disease), typical inflammatory, T-lymphocyte immune reaction is no longer observed. Instead, the CD4+ T-regulatory cells that were trained on the m1ψ antigen release several cell signals that suppress this immune response. Examination of the myelinating tissues within these animals show no reduction in normal myelination in contrast to the untreated animals, indicating that vaccination is protective against induction of demyelinating disease, at least using the established EAE gene-induced disease models (MOG35-55 or PLP139-151).
Pre-clinical Efficacy Data
To address the question of whether such vaccination could halt demyelinating autoreactivity in animals where model disease had already been established, the authors administered the m1ψ-modified mRNA vaccine at different time points after induction of the two different reactive antigens in murine EAE disease models. At earlier time points, mice do not show clinical symptoms, although their myelinated tissues show measurable signs of inflammatory response and degradation. In mice treated at earlier time points, disease progression is halted and clinical symptoms do not develop. By later time points, clinical disease can be readily observed, beginning with loss of function in the tail and hind limbs. In mice treated at later stages, disease progression was halted and there was even a slight improvement in clinical motor symptoms, which the authors attributed to anti-inflammatory effects of disease treatment rather than reversal of demyelination.
Overcoming antigen target limitations
One interesting result of the paper is how the authors showed that targeting the exact auto-antigen responsible for disease is not necessary to yield therapeutic success, so long as the antigen used in the vaccine is expressed in the same tissue as the disease-causing auto-antigen. This is very important because the cause of MS in humans is not well understood, and may vary substantially from person to person. If mRNA treatment is not dependent on a precise understanding of the auto-antigens for each patient, the technology has greater promise as a treatment since they may not require individualization.
The authors show this “bystander tolerance” effect by inducing one murine disease model for EAE using a known gene and then treated using the m1ψ coded mRNA vaccine for a different gene that induces an alternate EAE disease model. Despite the fact that the resulting T-regulatory cell populations were response-coded to the mRNA antigen instead of the disease-causing auto-antigen, this treatment successfully silenced auto reactive cell activity, showing similar efficacy in preventing and halting disease progression compared to mRNA vaccination using the disease-inducing auto-antigen. Because the resulting T-regulatory cell populations populate the same tissues where auto-antigen is expressed, the authors call this effect “bystander tolerance”, and posit that precise understanding of auto-antigens that cause MS or other auto-immune diseases in humans may not be needed if an antigen in the tissue of interest (for example, in the spinal cord and brain) can be identified and used to expand a population of T-regulatory cells capable of suppressing auto-immune response.
The authors further demonstrated that the T-regulatory cell populations that arose from mRNA auto-antigen vaccination did not suppress T-lymphocyte or inflammatory response to established models of foreign pathogens, providing promising data indicating that such a vaccine and resultant bystander tolerance is less likely to compromise the immune system beyond auto-antigens implicated in auto-immune disease. However, more study is needed to determine the extent of immune bystander effects in the specific tissues, both to understand the limits of bystander tolerance (e.g. how similar must auto-antigens be to elicit immune suppression?), and the extent to which bystander tolerance may compromise healthy immune function (e.g how does this make a patient more susceptible to infection).
While the promise of this mRNA vaccine approach to auto-immune disease is immense, BioNTech’s success must be caveated: These studies were performed using lab mice, with the mRNA “vaccine” derived from an antigen specifically selected due to its ability to induce EAE demyelinating disease similar but not identical to clinical MS in humans. Comparatively, our understanding of the auto-antigens that cause MS in humans is significantly lower than the genetic disease models used in this study, and this theme extends to most auto-immune conditions. Human immune systems still differ substantially from rodents, and this complexity could impact the effectiveness of the mRNA vaccine approach in humans, or limit the efficacy of so-called bystander tolerance. Still, what makes these mRNA approaches so exciting is their reliance on basic chemistry which is largely conserved between mammals, and therefore the breadth of disease which, in theory, can be addressed using the approach, if validated. In our opinion, the COVID-funded innovation happening in the mRNA community is one of the spaces that makes the future of biotech so promising.
“We believe that in 15 years, one-third of all newly approved drugs will be based on mRNA” – BioNTech CEO Uğur Şahin
Post Script: Why we believe BioNTech’s COVID-19 vaccine revenue will remain steady
Given that the BNT162b2 vaccine is BioNTech’s only approved drug, the future of their COVID-19 vaccine has understandably become investors’ main area of focus. For several reasons we list below, we feel this revenue stream to be durable in the near- to mid-term.
A booster dose of BNT162b2 was shown to significantly reduce risk of infection, hospitalization, and death vs. the Omicron variant
COVID-19 vaccines are likely to become an annual, multi-variant (& potential flu combo) booster
BioNTech’s Omicron-specific booster candidate has entered human trials
mRNA technology can respond quickly to new variants of COVID-19 and flu. Pre-approval (similar to annual flu vaccines) may be granted for seasonal vaccine updates longer-term
PFE partnership extends to future COVID-19 and Flu vaccines
Combined production will be 4B doses in 2022
Access to BIONTECH’s vaccine has been limited in most global markets due to western demand, while these sales are not locked in (therefore there is no guidance from BIONTECH to expect them) we would be surprised if demand does not continue to exceed production capacity through 2023
WHO, German, & US leaders, among others advocate for donation of vaccines to countries in need in order to rein in pandemic
€6.1B revenue in Q3 2021 alone
We see BIONTECH’s continued close relationship with Pfizer to be a market advantage. Given the widespread economic disruption of the Omicron variant, we believe in the continued success of one of the world’s most popular vaccine manufacturers.
Liability Release and Disclaimer. We are not a registered broker / dealer or an Investment Adviser as defined U.S. Investment Advisers Act of 1940 as amended. We do not offer or participate in offers of securities for sale or provide investment advice to others. This article is not intended to constitute (1) an offer to sell or buy, or a solicitation of an offer to sell or buy securities from the us; (2) investment advice or an offer to provide such advice; or (3) a basis for making any investment decision.
Information included in written reports, advice (oral or written), and other materials delivered as part of this article is believed, but not guaranteed, to be reliable, accurate and complete. Any opinions or estimates expressed in the Information reflect a judgment made as of this date and are subject to change. The Information is for informational purposes only and presented “as-is.” Accordingly, we do not make any representations, warranties, or guarantees with respect to the Information or any action taken or not taken in reliance thereon. The reader accepts the risk of loss in relying on the Information, and agrees to release and waive any claims for direct or consequential damages (including trading losses) arising out of its reliance on the Information. In no event will we be liable for any claims, losses (including trading losses), costs or damages of any kind, including direct, indirect, punitive, exemplary, incidental, special or, consequential damages, arising out of or in any way connected with the Information. Reader assumes the sole responsibility of evaluating the merits and risks associated with the use of any Information before making any decisions based on the Information. This limitation of liability applies regardless of any negligence or gross negligence.
Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, Akilli-Öztürk Ö, Kranz LM, Berger H, Petschenka J, Diken M, Kreiter S, Yogev N, Waisman A, Karikó K, Türeci Ö, Sahin U. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021 Jan 8;371(6525):145-153. doi: 10.1126/science.aay3638. PMID: 33414215.
https://www.science.org/doi/10.1126/science.aay3638?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
Wardell, C.M., Levings, M.K. mRNA vaccines take on immune tolerance. Nat Biotechnol 39, 419–421 (2021). https://doi.org/10.1038/s41587-021-00880-0
https://www.nature.com/articles/s41587-021-00880-0
Note: uridine corresponds to uracil bases in RNA which are analogous to the Thymine base in DNA, which code for different amino acids using triplet combinations of A, C, T, or G nucleotides
Oliwia Andries, Séan Mc Cafferty, Stefaan C. De Smedt, Ron Weiss, Niek N. Sanders, Tasuku Kitada, N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice, Journal of Controlled Release, Volume 217, 2015, Pages 337-344, ISSN 0168-3659, https://doi.org/10.1016/j.jconrel.2015.08.051.
https://www.sciencedirect.com/science/article/pii/S0168365915300948
Parr CJC, Wada S, Kotake K, et al. N 1-Methylpseudouridine substitution enhances the performance of synthetic mRNA switches in cells. Nucleic Acids Res. 2020;48(6):e35. doi:10.1093/nar/gkaa070
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7102939/
Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005 Aug;23(2):165-75. doi: 10.1016/j.immuni.2005.06.008. PMID: 16111635.
https://www.cell.com/immunity/fulltext/S1074-7613(05)00211-6?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1074761305002116%3Fshowall%3Dtrue